The Medicines and Healthcare products Regulatory Agency (MHRA) recently published their latest Good Pharmacovigilance Practice (GPvP) inspection metrics covering the period April 2018 to March 2019. Within this period a total of 18 inspections of Marketing Authorization Holders (MAHs) had been carried out, compared with 22 in 2017-2018.
MHRA GPvP Inspection Metrics Report : 2018/19
Interestingly, 50% of all inspections conducted during the reporting period included a remote inspection element (9). In addition, one inspection was conducted entirely remotely as part of a MHRA pilot project for conducting GPvP inspections remotely.
Of the 18 inspections that were conducted in the period, a total of 120 (4 critical, 78 major and 38 minor) findings were identified, compared to 162 findings in the previous year (4 critical, 89 major and 69 minor findings).
Although variability in the categorization of findings does not enable a direct comparison to previous years, a comparison of the general outcomes can be made.
The four critical findings identified in the current reporting period related to risk management (two), quality management system and provision of information for inspections. While risk management and quality management system also featured as critical findings in the previous reporting period, provision of information for inspections represents a new entry in this metric category.
The two critical risk management findings in the reporting period related to implementation of updated patient information leaflets and additional risk minimization measures.
The most frequently reported major finding categories were risk management (21% versus 25% in the previous period), followed by ongoing safety evaluation (19% vs 18%), provision of information for supervisions by NCAs (17% vs 16%), quality management system (15% vs 21%), management of adverse drug reactions (14% vs 12%) and collection of adverse drug reactions (12% vs 7%).
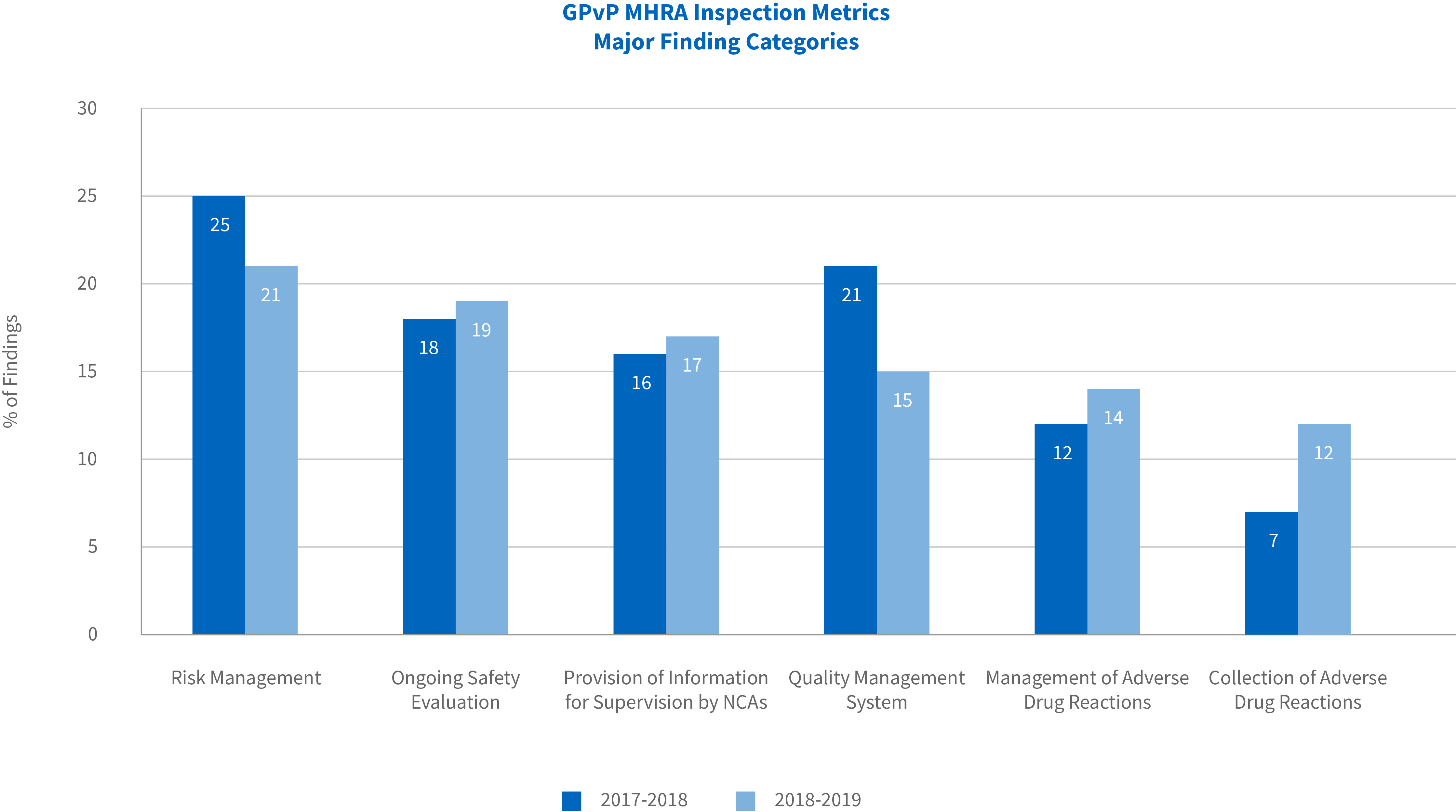
There was a reduction in the proportion of major findings related to risk management and quality management system, compared with the previous reporting period. However, an increase in major findings issued for core pharmacovigilance activities was noted with a rise in findings related to the collection of adverse drug reactions and the management of adverse drug reactions.
Of the 38 minor findings identified in the reporting period, the majority were related to collection and collation of adverse drug reactions (21% versus 9% in previous year), followed by quality management system (18% v 26%), ongoing safety evaluation (16% v 14%), risk management (16% v 14%) and management of adverse drug reactions (13% v 17%). The most noteworthy of the minor findings was the 12% increase in relation to collection and collation of adverse drug reactions.
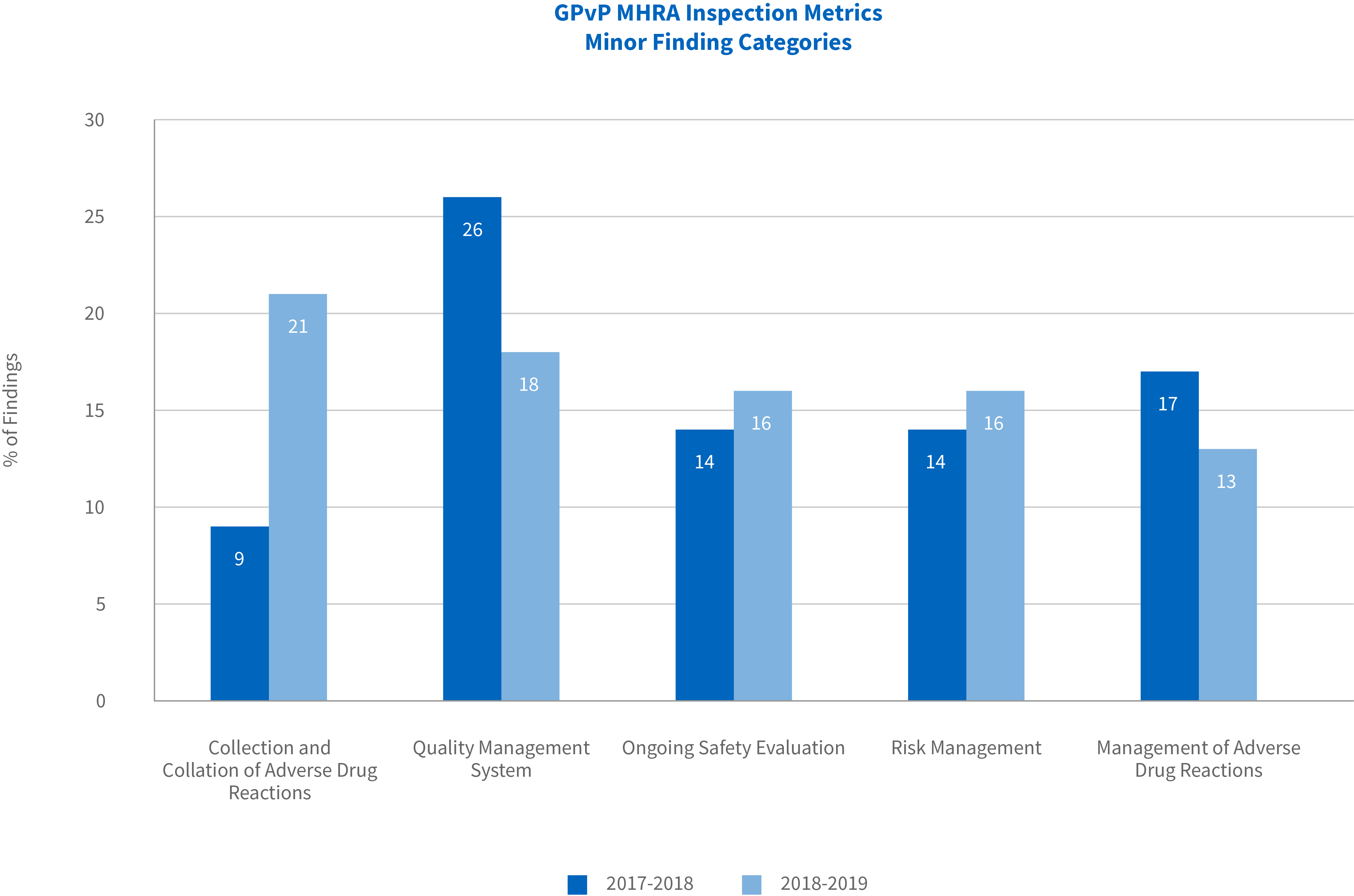
Overall for the reporting period, the highest proportion of inspection findings were in the category of risk management and primarily related to risk minimization measures. Ongoing safety evaluation findings contributed the second highest proportion of all findings, although for the first time since 2012-2013 there were no critical findings in this category.
A reduction was noted in major findings related to risk management and quality management system while there was a notable increase in major and minor findings related to the core pharmacovigilance activity of collection and collation of adverse drug reactions.
In summary, the latest MHRA GPvP Inspection metrics provide an interesting insight into the challenges MAHs continues to face in certain areas. To enable continuous improvement and maintain compliance in a proactive manner, auditing will continue to be a powerful tool at MAHs disposal to periodically review their PV system and that of their business partners and service providers.
At ADAMAS, we are very conscious that even in these difficult times our Clients’ have an ongoing need for compliance oversight and current travel restrictions make this very challenging. ADAMAS is well placed to assist with conducting audits and quality assessments remotely, over recent years our Consultants have gained wide experience with their conduct and successful delivery.
If you would like to learn more about how we can utilise our extensive experience in this area to support your compliance activities, please feel free to call us on +44 (0)1344 751 210 in Europe, +1-919-341-3361 in the US; or email us at info@adamasconsulting.com.
In addition, please check out our dedicated COVID-19 Series webpage, to learn about our remote service offerings.
The ADAMAS Team