The Guideline for Good Clinical Practice (GCP) E6(R1) has been undergoing revision since Jun-15 and the revised guidance, the Integrated addendum E6(R2) (‘the addendum’) is planned for finalization soon.
While the addendum includes few additions to investigator responsibilities, the majority of the revisions are related to the sponsor’s responsibilities, particularly to the sponsor’s quality management systems (QMS) and monitoring.
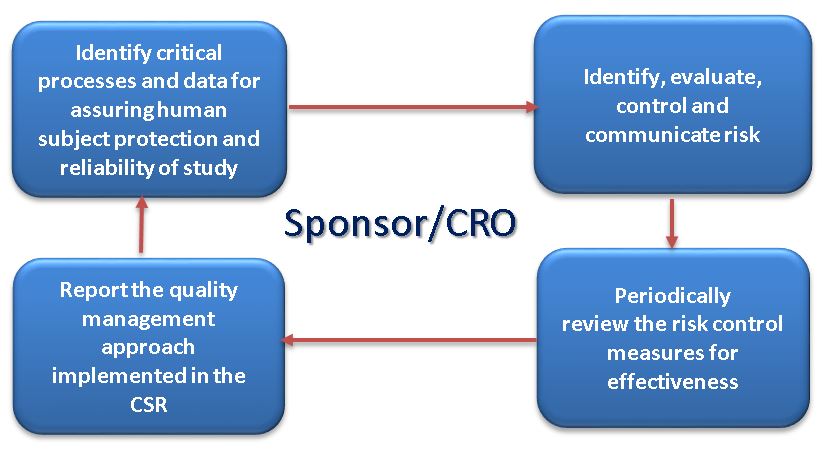
New section 5.0, Quality Management has been included and consequently the sponsor/CRO companies conducting clinical trials will need to ensure that their QMS supports the requirements of identifying, evaluating, controlling and communicating risks to the critical processes and the data:
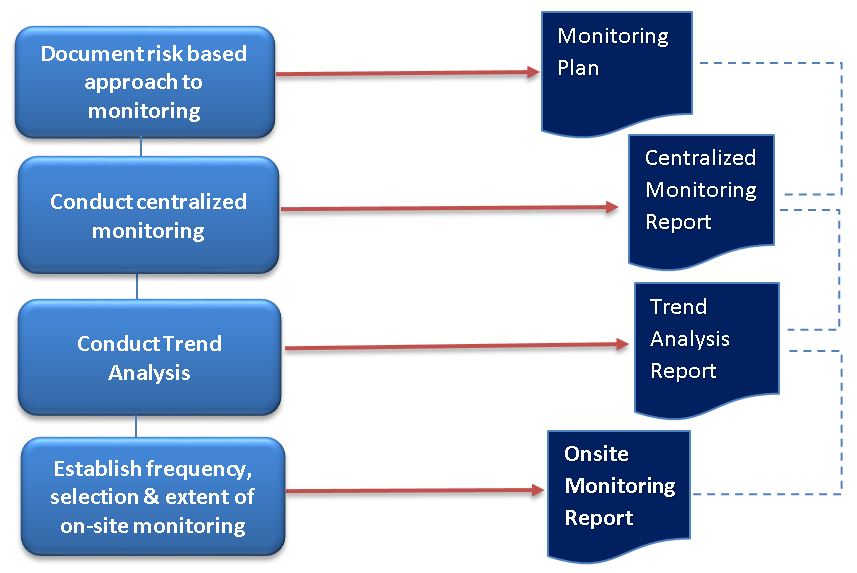
Section 5.18.3 includes a significant addendum to the extent and nature of monitoring. The sponsor/CROs conducting clinical trials will need to ensure that their standard operating procedures (SOPs) on monitoring supports a risk based approach.
 Furthermore, section 5.5.3 now includes the guidance that the sponsor/CROs handling or capturing electronic data for clinical trials will need to ensure that their SOPs for electronic data processing and reporting systems includes provision for:
Furthermore, section 5.5.3 now includes the guidance that the sponsor/CROs handling or capturing electronic data for clinical trials will need to ensure that their SOPs for electronic data processing and reporting systems includes provision for:
- Computer system validation (CSV)
- Functionality testing
- Data collection and handling
- System maintenance
- System security measures
- Change control
- Data back-up and recovery
- Contingency planning
- Decommissioning
- Training on the use of the computerised systems
Last but not the least, section 5.20 Noncompliance requires that the sponsor/CROs will need to ensure that their auditing SOPs include provision for:
- Conducting root cause analysis
- Development and implementation of corrective and preventive actions (CAPA)
- Reporting serious breaches to the regulatory authorities
CONCLUSION
Sponsor/CRO companies should conduct a gap analysis of their current systems and procedures and implement adequate CAPA to ensure compliance to the ICH GCP E6(R2) Addendum
If you would like us to conduct a gap analysis for you contact shehnaz.vakharia@adamasconsulting.com

